Atoms and Equations
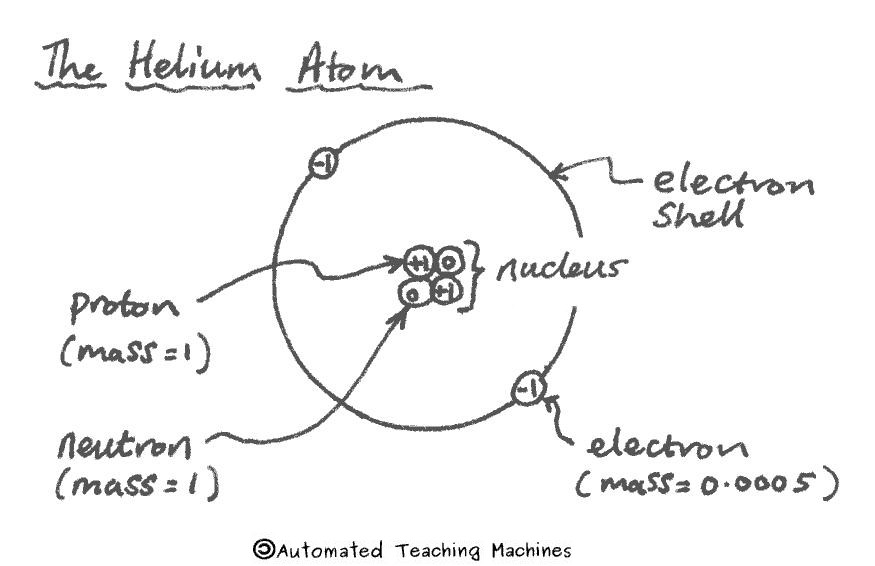
An atom has a small central nucleus with electrons orbiting around it in shells. Protons have a positive charge, the electron is negative and the neutron has no charge (it is neutral). Protons and neutrons both have a relative mass of 1 whereas the the electron is 2000 times lighter and only has a relative mass of 0.0005
The chlorine molecule Cl2 contains two chlorine atoms as indicated by the number 2. The equation 2H2 + O2 -> 2H2O is balanced. This means that the number of reactant atoms of each element is equal to the number of product atoms.