Acids and Alkalis
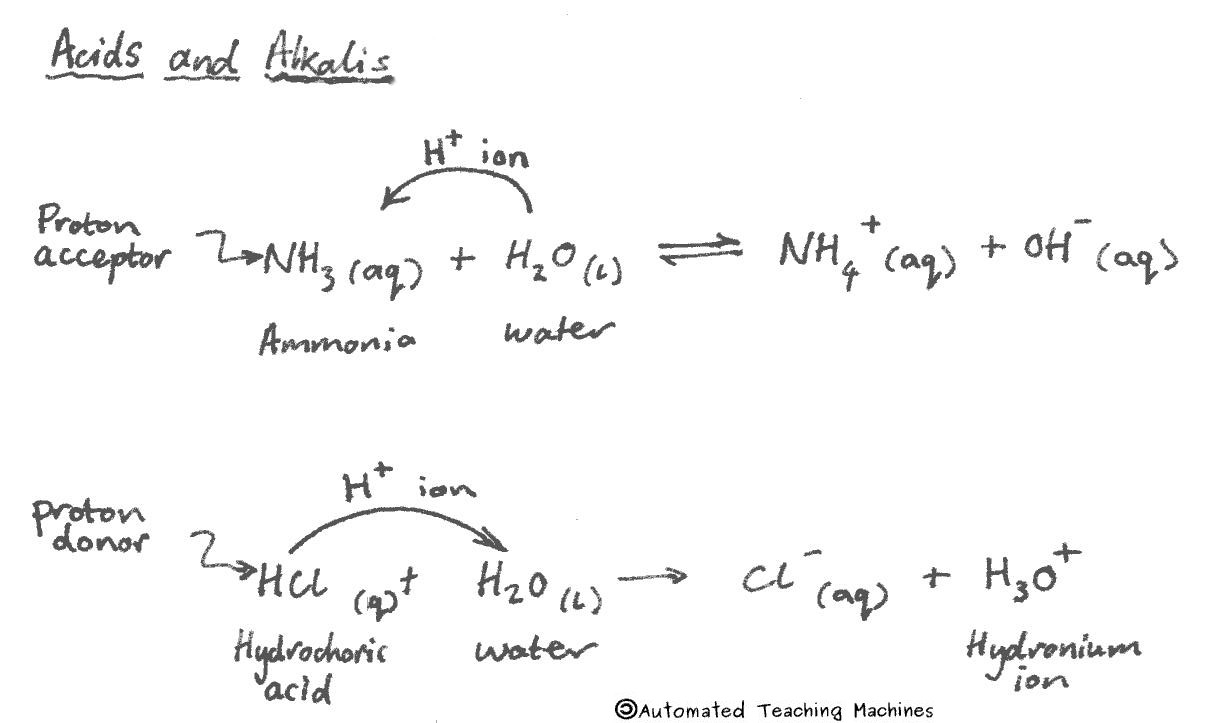
Acids have a pH less than 7 and will turn universal indicator red, orange or yellow, and litmus paper red. Acids are proton donors because they produce hydrogen ions (H+) in water.
Alkalis have a pH greater than 7 and will turn universal indicator blue or purple, and litmus blue. Alkalis are proton acceptors because they produce hydroxide ions (OH-) in water.
A neutralisation reaction takes place when acids and alkalis react to produce salt and water. Neutral substances have a pH of 7. Bases neutralise acids and those that dissolve in water are called alkalis.